Kumashure uye Dhizaini Yekudzidza
Retatrutide (LY3437943) inyowani imwe-peptide mushonga unoshandamatatu receptors panguva imwe chete: GIP, GLP-1, uye glucagon. Kuti uongorore kushanda kwayo uye kuchengeteka kune vanhu vane kufutisa asi vasina chirwere cheshuga, chikamu chechipiri, randomized, double-blind, placebo-controlled controlled trial yakaitwa (NCT04881760). Zvose zve338 vatori vechikamuine BMI ≥30, kana ≥27 ine kanenge kamwe-yakabatana-inorema comorbidity, yakaitwa randomized kuti igamuchire placebo kana retatrutide (1 mg, 4 mg ine maviri titration schedules, 8 mg ine maviri titration schedules, kana 12 mg) inotungamirirwa kamwe vhiki nevhiki ne subcutaneous jekiseni kwevhiki dze48. Theprimary endpointyakanga iri shanduko yeperesenti yehuremu hwemuviri pamasvondo e24, nemagumo echipiri anosanganisira kuchinja kwehuremu pamavhiki e48 uye zvikamu zvehuremu-kurasikirwa (≥5%, ≥10%, ≥15%).
Key Migumisiro
-
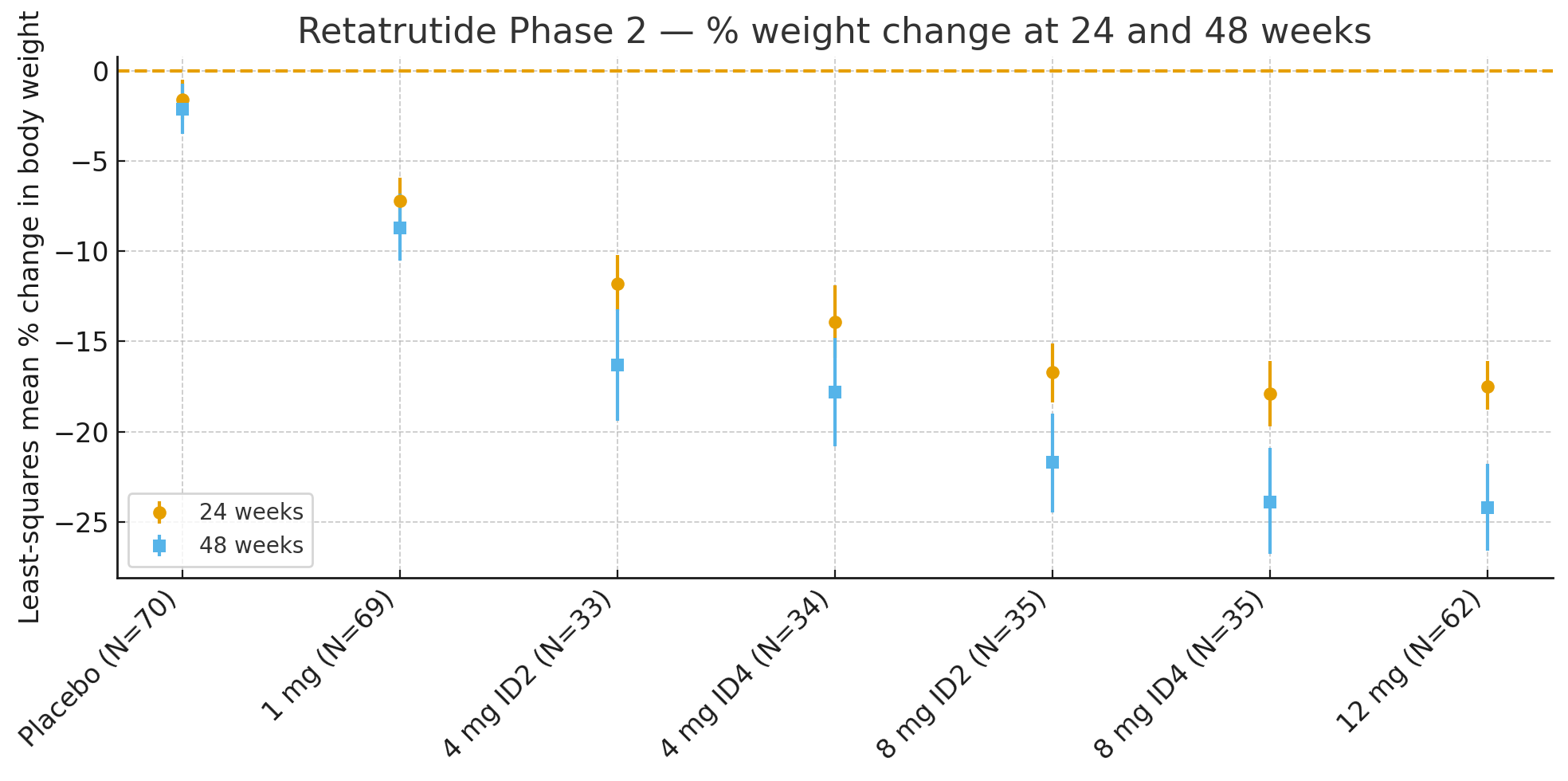
mavhiki makumi maviri nemana: Zvishoma-zvikwere zvinoreva muzana shanduko muhuremu hwemuviri maererano nekutanga yaive
-
Nzvimbo: −1.6%
-
1 mg: -7.2%
-
4 mg (yakasanganiswa): -12.9%
-
8 mg (yakasanganiswa): -17.3%
-
12 mg: -17.5%
-
-
48 mavhiki: Muzana shanduko yehuremu hwemuviri yaive
-
Nzvimbo: −2.1%
-
1 mg: -8.7%
-
4 mg (yakasanganiswa): -17.1%
-
8 mg (yakasanganiswa): -22.8%
-
12 mg: -24.2%
-
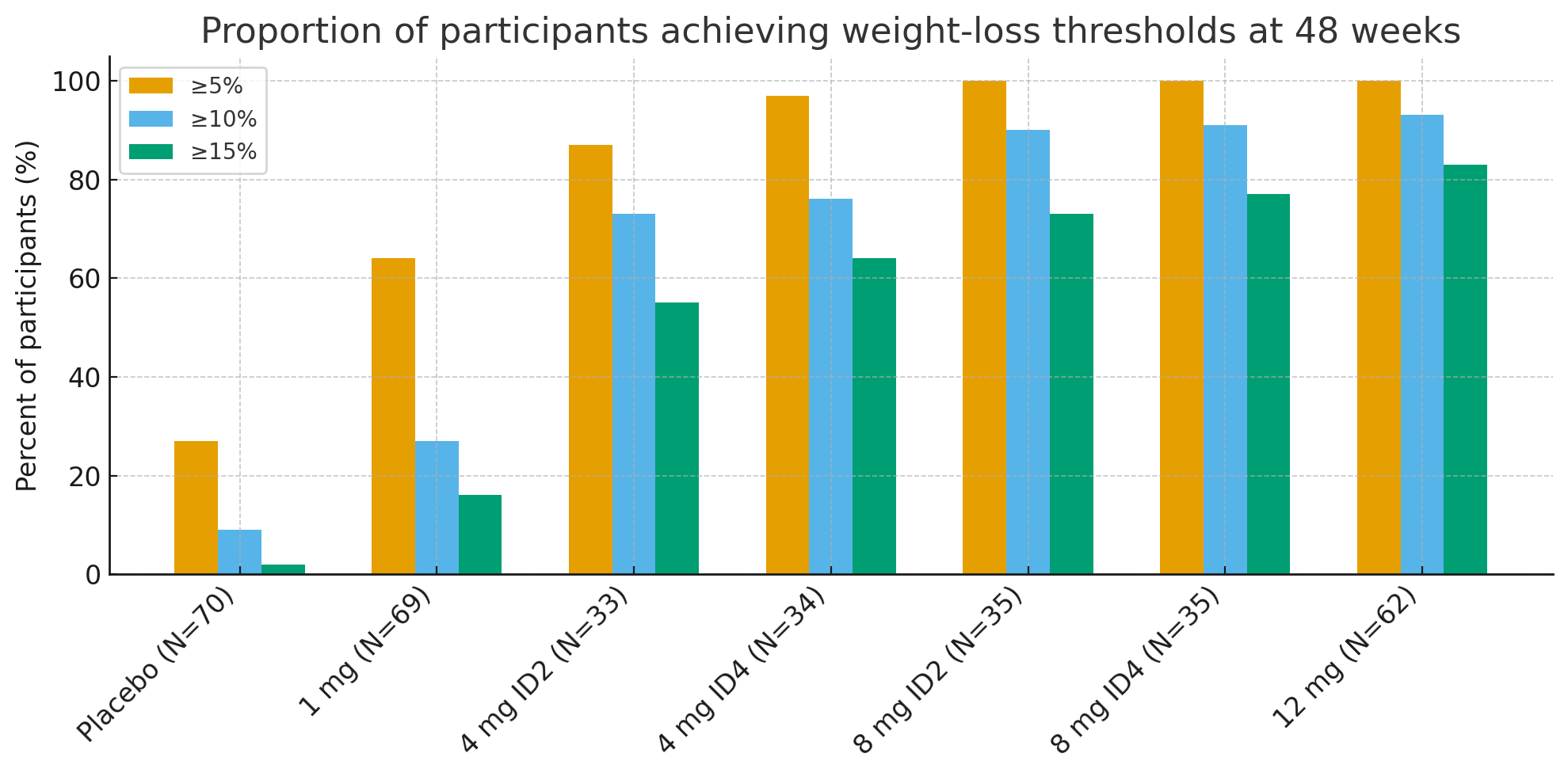
Pamasvondo e48, chiyero chevatori vechikamu vanowana zvinorehwa nekiriniki-kurasikirwa zvikumbaridzo zvaishamisa:
-
≥5% kurasikirwa uremu: 27% ne placebo vs. 92-100% mumapoka anoshanda
-
≥10%: 9% ne placebo vs. 73-93% mumapoka anoshanda
-
≥15%: 2% ne placebo vs. 55-83% mumapoka anoshanda
Muboka re12 mg, kusvika26% yevatori vechikamu vakarasikirwa ≥30% yehuremu hwavo hwekutanga, hukuru hwekurasikirwa nehuremu hunofananidzwa nekuvhiyiwa kwebariatric.
Safety
Zviitiko zvakanyanya kuipa zvaive zvedumbu (kusvotwa, kurutsa, manyoka), kazhinji zvinyoro kusvika pakati nepakati uye zvine chekuita nedosi. Lower kutanga doses (2 mg titration) yakaderedza zviitiko izvi. Kuwedzera kwakabatana nedose mukurova kwemoyo kwakaonekwa, kukwira pavhiki 24, ndokuzoderera. Discontinuation rates kubva pa6-16% pamapoka akashanda, akati wandei kupfuura placebo.
Mhedziso
Muvakuru vane kufutisa vasina chirwere cheshuga, vhiki nevhiki subcutaneous retatrutide kwemavhiki makumi mana nemasere inogadzirwakuderera kukuru, kunoenderana nedosi muhuremu hwemuviri(kusvika ~ 24% zvinoreva kurasikirwa pachiyero chepamusoro), pamwe nekuvandudzwa kwemakaki e cardiometabolic. Zviitiko zvemudumbu zvakashata zvaive zvakajairika asi zvichigoneka netitration. Izvi zvakawanikwa zvechikamu chechipiri zvinoratidza kuti retatrutide inogona kumiririra bhenji nyowani yekurapa yekufutisa, ichimirira kusimbiswa mune yakakura, yenguva refu yechikamu 3 miedzo.
Nguva yekutumira: Sep-28-2025