Chiratidzo (kushandiswa kwakatenderwa): Muna 2019, iyo FDA yakabvumira kuti irapwe yakawanikwa, generalized hypoactive sex chishuwo chirwere (HSDD) muvakadzi vepremenopausal kana mamiriro acho achikonzera kushushikana kwakanyanya uye kwete nekuda kwemamwe mamiriro ekurapa / epfungwa kana mhedzisiro yezvinodhaka.
Nzira Yekuita
PT-141 ndeye melanocortin receptor agonist (kunyanya MC4 receptor) inogadzirisa chishuwo chepabonde kuburikidza nepakati tsinga system nzira.
Kusiyana nePDE5 inhibitors (semuenzaniso, sildenafil), iyo inonyanya kukanganisa tsinga dzeropa, PT-141 inoshanda nechepakati kuti iite zvekukurudzira zvepabonde uye kumutsa.
Pharmacology & Dosing
Kutonga: Subcutaneous jekiseni, sezvinodiwa (pane-inoda).
Nhamba yakagamuchirwa: 1.75 mg sc
Pharmacokinetics:
Tmax ≈ ~ maminitsi makumi matanhatu
t½ ≈ maawa 2–3
Mhedzisiro inogona kutora maawa akati wandei, mune mamwe mishumo kusvika ~ maawa gumi nematanhatu.
Clinical Efficacy (Phase III Miedzo - RECONNECT, mavhiki e24, RCTs)
Mamiriro ekutanga:
Mukadzi Sexual Function Index-Desire domain (FSFI-D)
Chiyero cheKunetseka Kwepabonde Kwevakadzi (FSDS-DAO)
Mhedzisiro yakakosha (zvidzidzo zvakabatanidzwa 301 + 302):
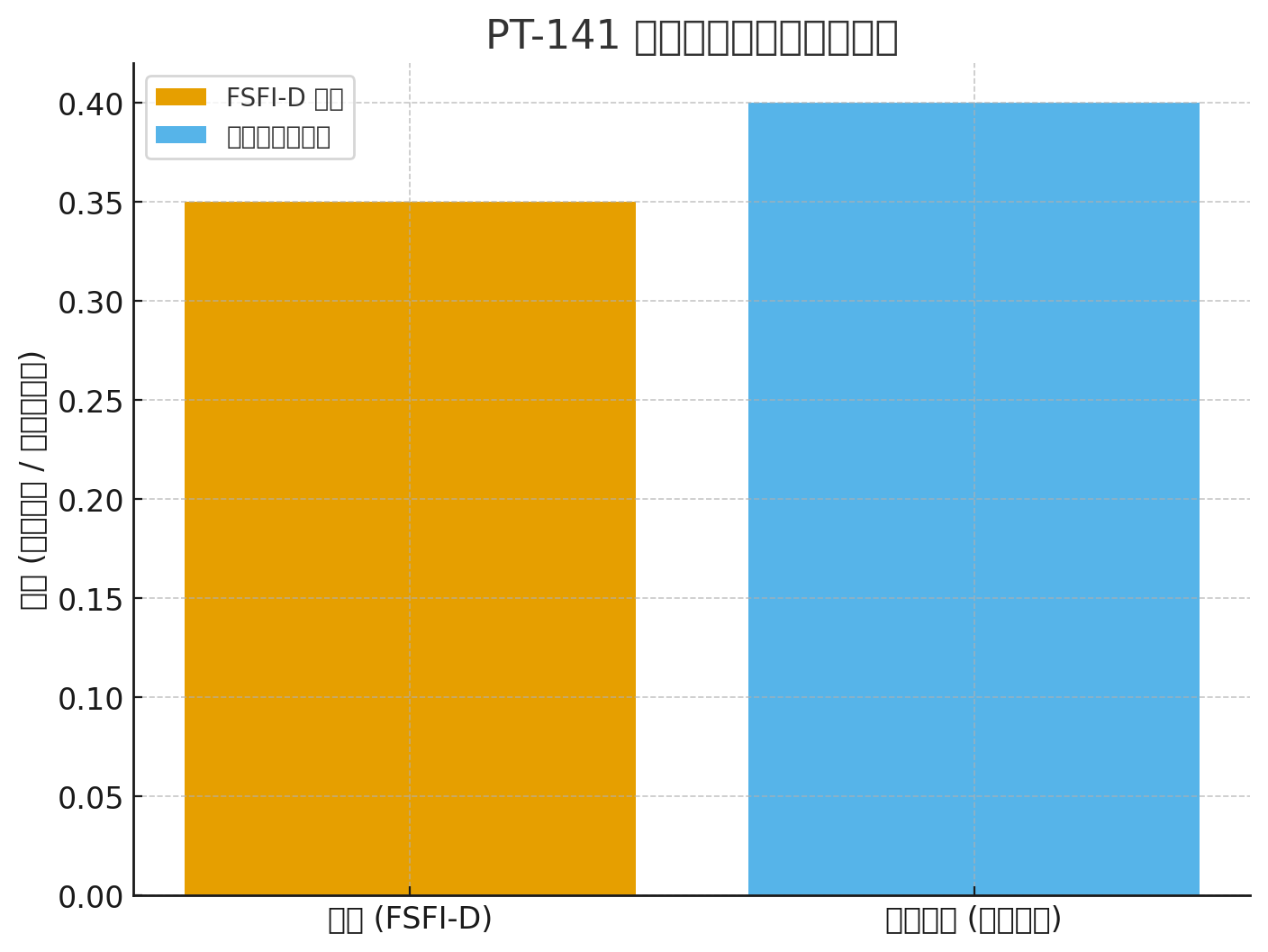
FSFI-D kunatsiridza: +0.35 vs placebo (P <0.001)
FSDS-DAO kudzikisa zvibodzwa: -0.33 vs placebo (P <0.001)
Zvimwe zvinogumira: Migumisiro inotsigira (zvibodzwa zvebasa repabonde, kugutsikana-kwakashumwa nemurwere) zvakafambiswa zvakanaka, asi zviitiko zvebonde zvinogutsa (SSEs) hazvina kugara zvichiratidza misiyano yakakosha.
Zviitiko Zvakaipa (zvinowanzotaurwa mumiedzo)
Zvakajairwa (≥10%):
Nausea (~ 30-40%; kusvika ~ 40% yakataurwa mumiedzo)
Kutsvaira (≥10%)
Musoro wemusoro (≥10%)
Cardiovascular effects:
Kuwedzera kwenguva pfupi muropa uye kuchinja kwehutano hwemwoyo kwakaonekwa, kazhinji kugadzirisa mukati memaawa mashomanana.
Contraindicated kana kushandiswa nekuchenjerera kune varwere vane isingadzoreki hypertension kana chirwere chemoyo.
Chiropa: Kashoma mishumo yenguva pfupi yechiropa enzyme yakakwira; mishumo isingawanzoitiki inoratidza kukuvara kwakanyanya kwechiropa, asi kwete kwakajairika.
Kuchengetedza Kwenguva Yakareba (Chidzidzo Chekuwedzera)
Chidzidzo che52-vhiki yakavhurika-label yekuwedzera yakawana kuvandudzwa kwechishuwo pasina masaini makuru ekuchengetedza.
Yenguva refu yekuchengetedza chimiro inoonekwa seyakatenderwa zvakanaka, nenyaya huru dzekushivirira dzichiri dzenguva pfupi dzakaipa mhedzisiro sekusvotwa.
Mazano Anokosha Ekushandisa
Huwandu hunotenderwa hushoma: Chete kune vakadzi vepamberi vane HSDD inowanikwa, yakazara.
Haisi kubvumidzwa zvakanyanya kuvarume (ED kana chishuwo chakaderera muvarume chinoramba chiri kuongorora).
Kuongororwa kwekuchengetedza kwakakosha: Hypertension, chirwere chemoyo, uye nhoroondo yechiropa inofanirwa kuongororwa isati yapihwa.
Quick Data Summary
FDA Mvumo: 2019 (Vyleesi).
Dose: 1.75 mg subcutaneous jekiseni, painoda.
PK: Tmax ~ 60 min; t½ 2–3 h; mhedzisiro inosvika ~ 16 h.
Kubudirira (Chikamu chechitatu, chakabatanidzwa):
FSFI-D: +0.35 (P <.001)
FSDS-DAO: −0.33 (P<.001)
Zviitiko zvakashata:
Nausea: kusvika ~ 40%
Kugeza: ≥10%
Musoro wemusoro: ≥10%
Transient BP inowedzera.
Kuenzanisa Tafura & Girafu (Pfupiso)
| Kudzidza / Data Type | Endpoint / Measure | Kukosha / Tsanangudzo |
|---|---|---|
| Chikamu chechitatu (301+302 zvakabatanidzwa) | FSFI-D (chido domain) | + 0.35 vs placebo (P <0.001); FSDS-DAO −0.33 |
| Zviitiko Zvakaipa | Nausea, flushing, musoro | Nausea ~ 30–40% (max ~ 40%); kupisa ≥10%; musoro ≥10% |
Nguva yekutumira: Sep-30-2025